Center for Advanced Cellular Therapies
Catalyzing the development of life-saving cancer treatments
- Client
- University of Pennsylvania Perelman School of Medicine and Novartis
- Location
- Philadelphia, Pennsylvania, United States
- Size
- 30,000 square feet
- Status
- Completed
- LEED Gold Certified
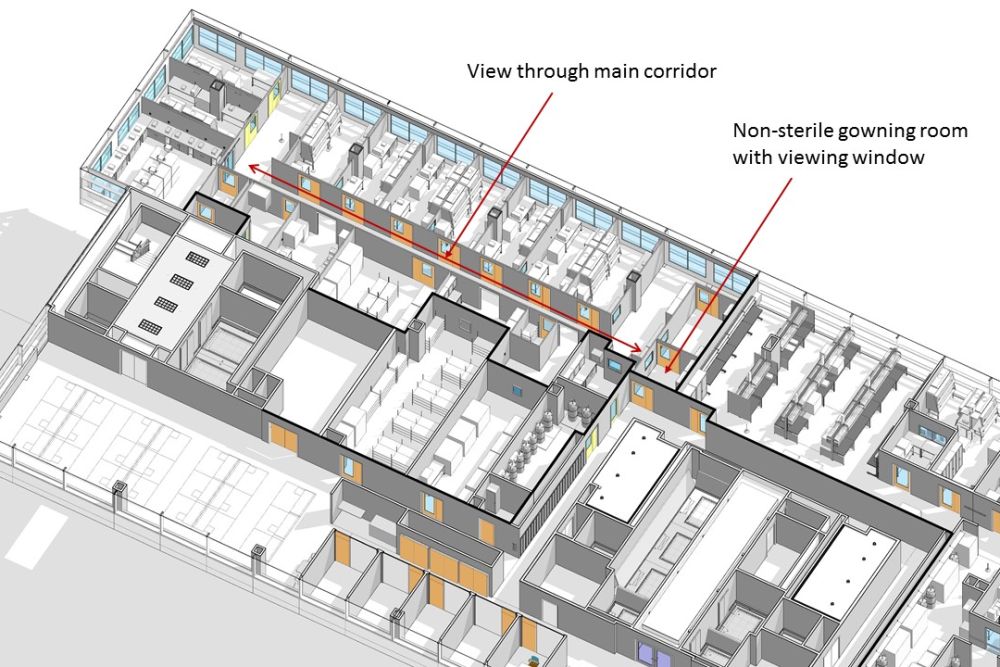
A former patient currently in remission from childhood leukemia for more than six years comes in for a visit to the Novartis-Penn Center for Advanced Cellular Therapies (CACT). Peering through the cleanroom viewing window, she is able to see where her treatment was created, while the researchers are also able to see the result of their life-saving work. For the best minds to solve some of medicine’s biggest challenges, the right environment can make all the difference.
This type of encounter happens on a regular basis at the CACT, where over 100 highly specialized researchers are developing groundbreaking immunotherapies that use patients’ own immune systems to fight—and oftentimes cure—their once incurable cancer. To create a critical mass of innovation and productivity at the facility, we designed the space to make researcher wellness, efficiency and satisfaction a top priority.
Our team included both laboratory designers and experts in workplace design and strategy. By improving the layout and organization of the laboratory environment, the optimized design reduces the time it takes to create the “hunter cells” for one patient by 50 percent. At the outset of this effort, it took an entire month to tackle this for each patient; now, it only takes two weeks which allows for quicker treatment delivery and larger development capacity.
By the numbers
25%
increase in programming efficiency by bringing all PI teams into the CACT.
50%
reduction in the time it takes to create the "hunter cells" for one patient.
400
The space has the capacity to manufacture cellular therapies for up to 400 patients per year.
100
highly specialized researchers are housed in the space.
What takes a research environment from good to great is the satisfaction of the researchers who use it. Floor-to-ceiling windows provide crucial daylight benefits often overlooked in traditional manufacturing laboratories. Not only does the center provide views to the outside, but it also contains windows that interconnect all adjacent suites, which opens the workspace even more and encourages collaboration.
The CACT also worked with our design team to install a window in the non-sterile change room so that former patients, various school groups, National Geographic reporters and even Joe Biden (who visited the facility) can see the incredible research happening within, and for the researchers to be reminded how their cutting-edge work is impacting the world. By working with designers to find safe and reasonable ways to put this science on display, researchers can enhance public perception; strengthen alliances with academic, professional and industrial organizations; and better facilitate dialogue with lawmakers and patient advocates.